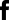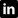In the field of 3D bioprinting, bioink development is one of the most challenging problems. The ideal bioink meets the biological, physical and mechanical requirements of the printing process. In terms of biology, bioinks must have biocompatibility, cytocompatibility and bioactivity. As such it must be
(1) Non-inflammatory and non-cytotoxic
(2) Provide an environment similar to natural extracellular mat
(3) Allow living cells to adhere and proliferate in an arrangem
Physically, the ink needs to have a low enough viscosity to not clog the printhead. At the same time, the bioink needs to provide sufficient mechanical strength and stiffness Therefore, we aim Celluid bioinks series to develop personalized scaffolds for repairing defective tissues, tissue regeneration, and even printing tissues or organs.
-

Bioink
-
3D Bioprinting of Scaffold
-
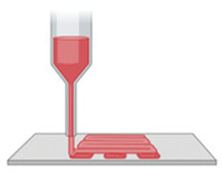
Healthy wound healing proceeds
-
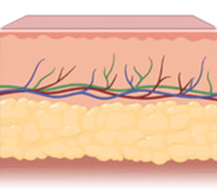
Scaffold application supports cell growth and tissue regeneration
-
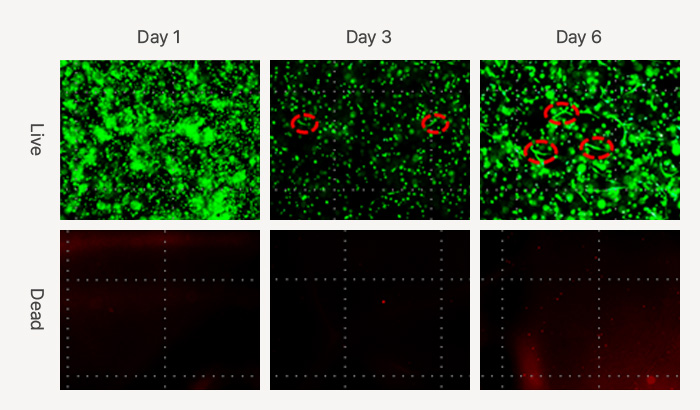
Live & dead images of HDFs (human dermal fibroblast) in Celluid GM for 6 days
(Green: viable cells, Red: dead cells)
-
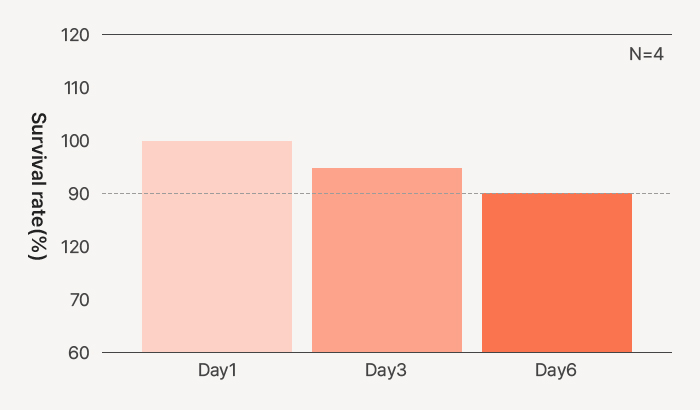
Survival rate (>95%) of HDFs in Celluid GM for 6 days
Currently, colorectal cancer is still the third leading cause of cancer-related mortality, and the incidence is rising. It is a long time since the researchers used cancer cell lines and animals as the study subject. However, these models possess various limitations to reflect the cancer progression in the human body.
Organoids have more clinical significance than cell lines, and they also bridge the gap between animal models and humans. Patient-derived organoids are three-dimensional cultures that simulate the tumor characteristics in vivo and recapitulate tumor cell heterogeneity. Therefore, the emergence of colorectal cancer organoids provides an unprecedented opportunity for colorectal cancer research.
Colorectal cancer organoids show great potential for application, especially preclinical drug screening and prediction of patient response to selected treatment options.
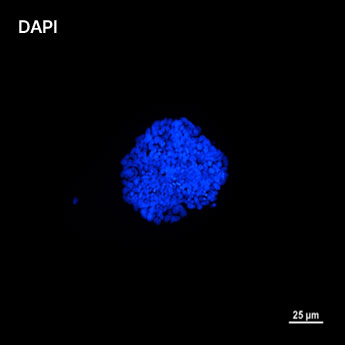
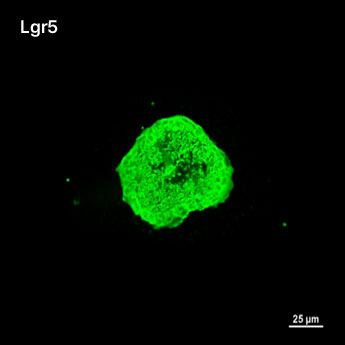
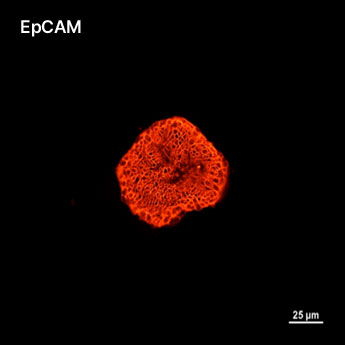
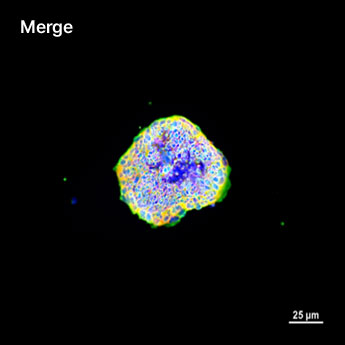
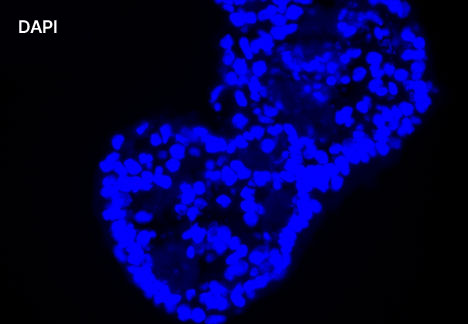
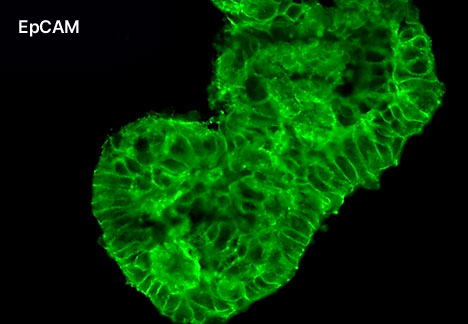
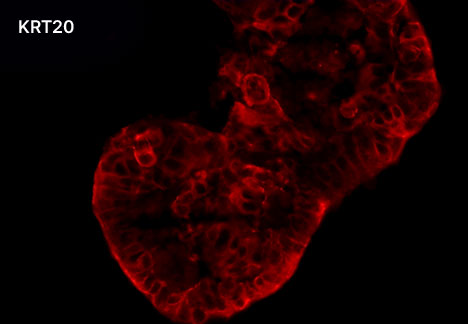
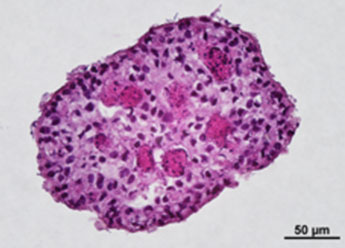
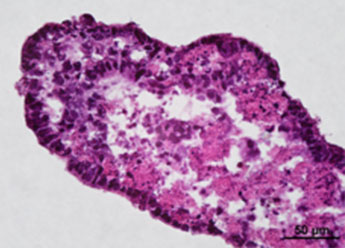
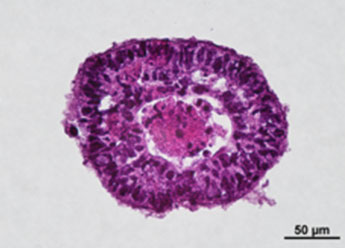
1. Fluorescent Characterization of Colorectal Cancer-Derived Cancer Stem Cells.
2. Fluorescent Characterization of Colorectal Cancer-Derived Cancer Stem Cell Organoids Differentiation.
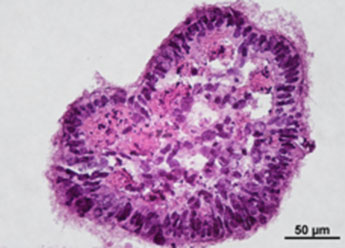
3. Histological Characterization of Differentiated Colorectal Cancer-Derived Cancer Stem Cell Organoids
-
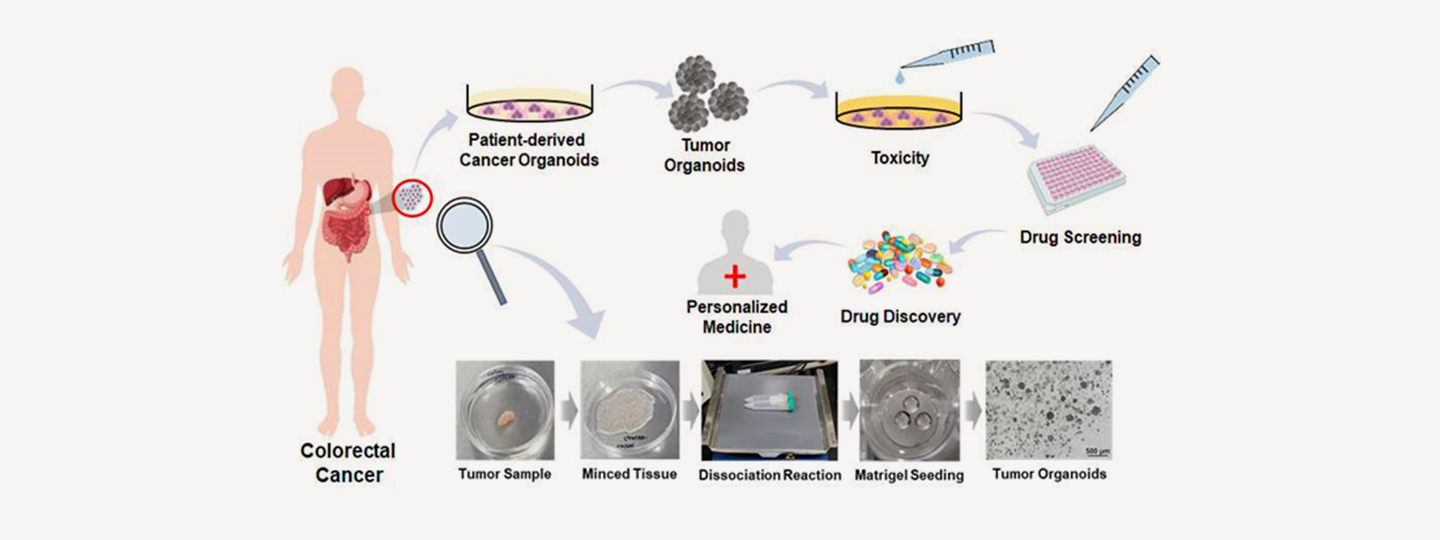
Schematic illustration: Potential applications of cancer organoids to improve treatment prediction and clinical applicability.
Induced pluripotent stem cells are an increasingly popular cell source for remodeling human disease. iPSCs are cells from any genetic background of the donor, with the ability to differentiate into almost any desired terminal cell type.
Currently, researchers are conducting a variety of studies using iPSCs in drug development, disease models, and transplantation, and the potential applications of iPSCs are enormous. These iPSCs have the ability to differentiate into a variety of cell types through their pluripotency and are used to study differentiation, tissue repair, disease pathogenesis, and drug discovery and development.
These induced pluripotent stem cells are characterized by the expression of a variety of genes, including the pluripotency genes Oct4, Nanog, SOX2, Klf4 and carry the provider’s genetic background, so they can carry patient-specific genetic traits for gene deficiency or overexpression in patients with genetic diseases.
Based on this ability of induced pluripotent stem cells, many researchers are currently conducting various studies, and our research team is conducting research and development to utilize induced pluripotent stem cells to create artificial skin models.
-
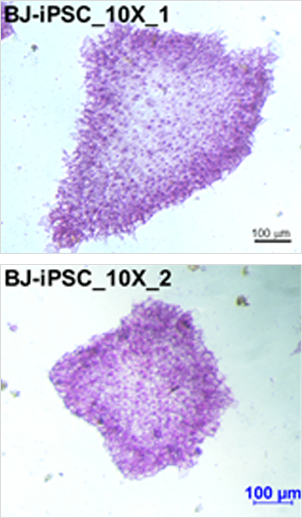
AP(alkaline phosphatase) staining
-
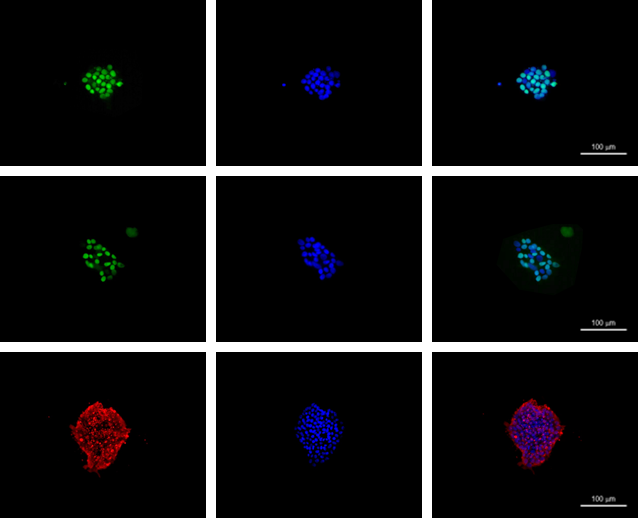
IF(immuno fluorescence) puripotency markers of hiPSCs EBs
-

Extraction of Human-Derived Stem Cells
-
Gene introduction (Oct4, SOX2, Klf4, c-Myc)
-

Dedifferentiation Induction
-

Induced Pluripotent Stem Cell Cultivation
Cell differentiation ability
Capable of Differentiating into All Types of Cells that Make up the Human Body
Cell proliferation ability
Almost unlimited proliferation
Immune rejection
Free from immune rejection through the use of autologous cells.
Scope of cell applications
Drug development, Cell therapy, Organoids, Drug screening, etc
Ethical concerns
No ethical problems using autologous adult tissue for research purposes within the scope of the study